57+ calculate the edge length of the unit cell of potassium.
Potassium metal has a body-centered cubic structure with all atoms at the lattice points. Web To calculate edge length in terms of r the equation is as follows.
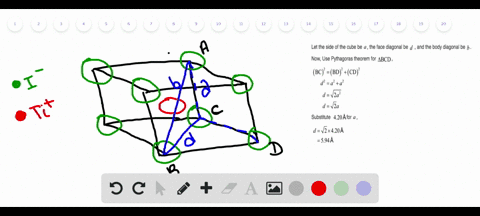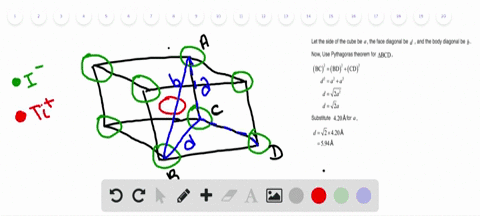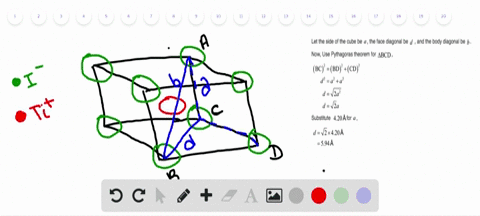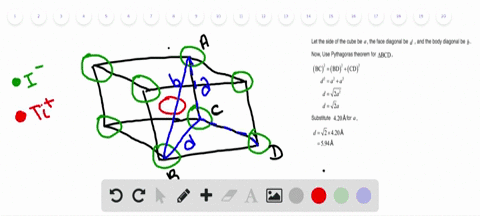
Solved Nah Crystallizes With The Same Crystal Structure As Nacl The Edge Length Of The Cubic Unit Cell Of Nah Is 4 880 A A Calculate The Ionic Radius Of H The Ionic
If the edge length of unit cell is 533 pm calculate the radius of potassium atom.

. Web Find Unit Cell Edge Length Given Density chemistNATE 240K subscribers 174K views 11 years ago If you have the density of a metal and know its crystal structure you can find its unit cell. 2 Atomic mass of K39 g mol1 NA 60221023 mol1 Solution For b Potassium metal crystallises in a bcc unit cell with edge length 542pm. Volume Metric volume Solvers Lessons Click here to see ALL.
We have edge length of unit. Edge length cm Now assume that. Web Calculate the density of potassium metal.
Metallic potassium has a body-centered cubic structure. Assuming anion-cation contact along the cell edge calculate the radius of the potassium ion. KBr or potassium bromide has density 275 g cm-3.
Sodium has a density of 0971 gcm 3 and crystallizes. Web What is the edge length of the unit cell. The density of the metal is 08560 gcm.
Trying to find the edge length of a unit cell. Web The edge length of the unit cell of KCl NaCl-like structure FCC is 628 Å. Solution Alpha polonium crystallizes in a simple cubic unit cell.
Potassium ionic sulfur dioxide molecular graphite covalent methane molecular. Web The edge length of the unit cell of alpha polonium is 336 pm. Body-centered Cubic Unit Cells Body-centered Cubic BCC unit cells indicate where the lattice points appear not only at the.
The edge length of its unit cell is 654 pm. The radius of the potassium ion is 133 Å. Web If the atomic radius of potassium is 227 pm find the edge length of the unit cell.
Palladium crystallizes with a face-centered cubic structure. The density of the metal is 0856gcm3. The radius of the chloride ion is 182 Å.
A Determine the radius of a polonium atom. Prove that KBr depicts face-centred cubic structure. Eight zinc atoms at the corners four zinc atoms at the faces and one at the center gives 4 whole Zn and four whole sulfur.
AND such a cubic cell contains FOUR formula units of ZnS which each have a formula mass of 9747 g mol1. Calculate the edge length a of a unit cell of calcium. Web Potassium metal has a body-centered cubic structure with one atom at each lattice point.
B Determine the density of alpha polonium. From this information and the atomic weight calculate the edge length of the unit cell. Metallic calcium crystallized in a face-centered cubic lattice and the atomic radius of calcium is 197Å.
2r An example of a Simple Cubic unit cell is Polonium. Web After evaluating we get the value of edge length of the cube to be 4 10-8 cm. A Two adjacent Po atoms contact each other so.
It has a density of 120 g c m 3 g cm3 g c m 3 a radius of 138 pm and a molar mass of 10642 gmol.
The Edge Length Of The Unit Cell Of Ta Is 330 6 Pm The Unit Cell Is Body Centred Cubic Tantalum Has A Density Of 16 69 G Cm 3 Sarthaks Econnect Largest Online Education Community
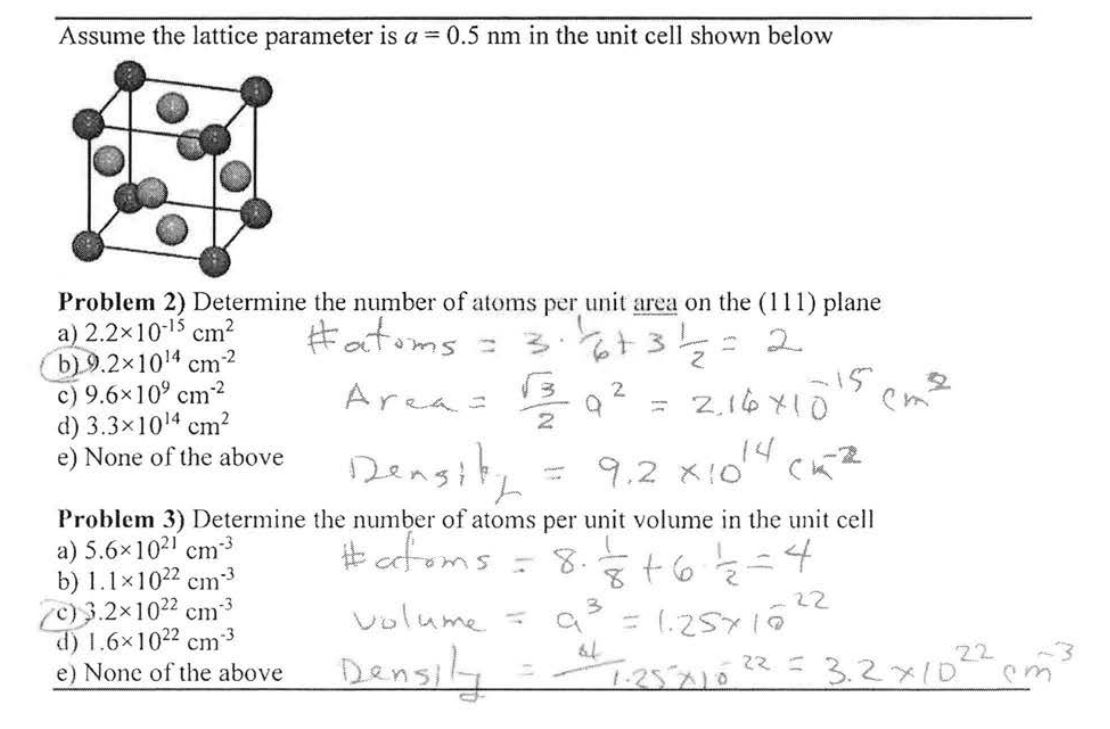
Solved Assume The Lattice Parameter Is A 0 5 Nm In The Unit Chegg Com

Schaum S Analytical Chemistry Pdf Pdf

The Edge Length Of The Unit Cell Of Kcl Fcc Is 6 28 A Assuming Anion Cation Contact Along The Cell Edge Calculate The Radius In A Of The Potassium Ion Rcl 1 8 A
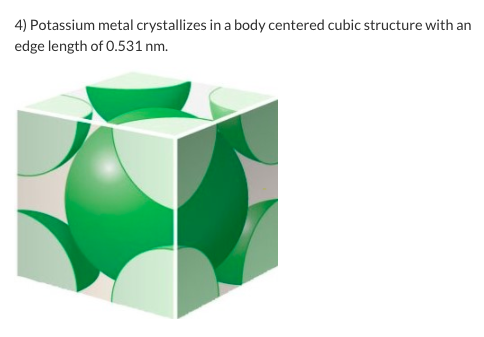
Solved 4 Potassium Metal Crystallizes In A Body Centered Chegg Com

Potassium Crystallizes In Body Centered Cubic Lattice With A Unit Cell Length A 5 2 A A What Is The Distance Betweenn Nearest Neighbourss B What Is The Distance Between Next Nearest
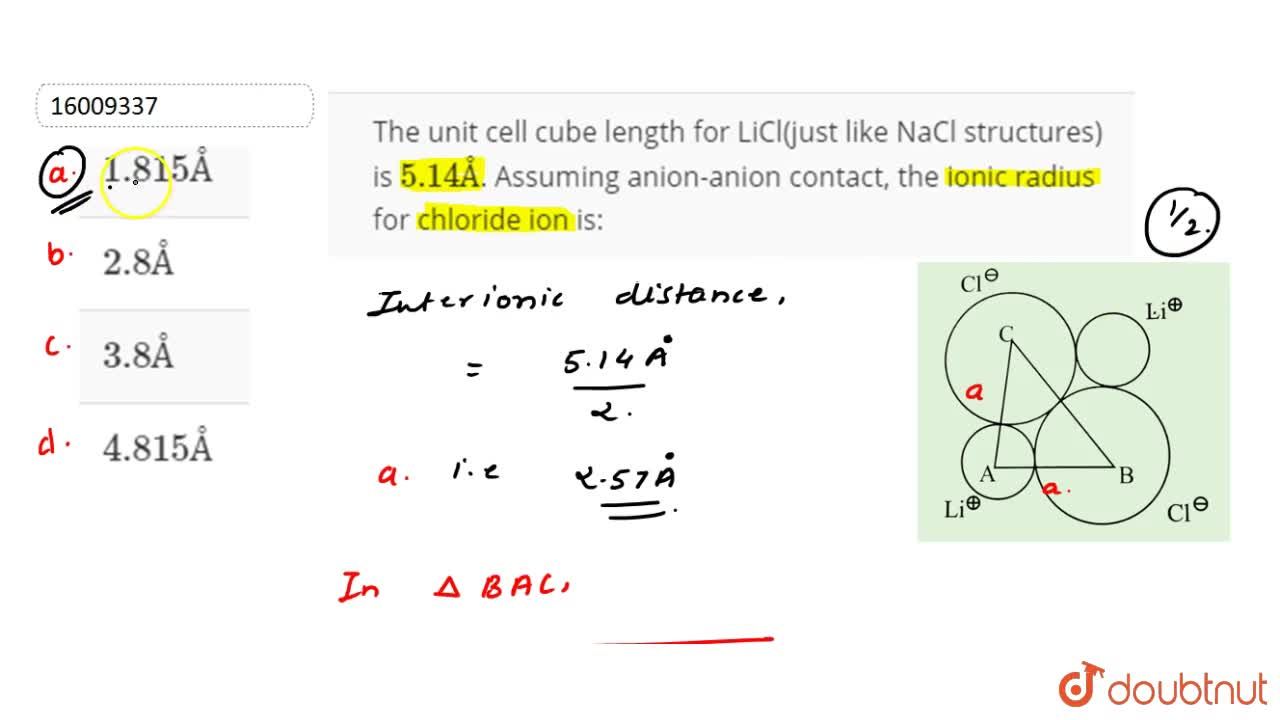
The Unit Cell Cube Length For Licl Just Like Nacl Structures Is 5 14a Assuming Anion Anion Contact The Ionic Radius For Chloride Ion Is

Potassium Metal Has Body Centred Cubic Crystal The Edge Length Of The Unit Cell Of The Crystal Is 0 542 Nm Determine The Radius Of K Atom And The Volume Occupied By The Atoms

How To Calculate The Edge Length Of The Unit Cell Neet Exam Study Guide Youtube
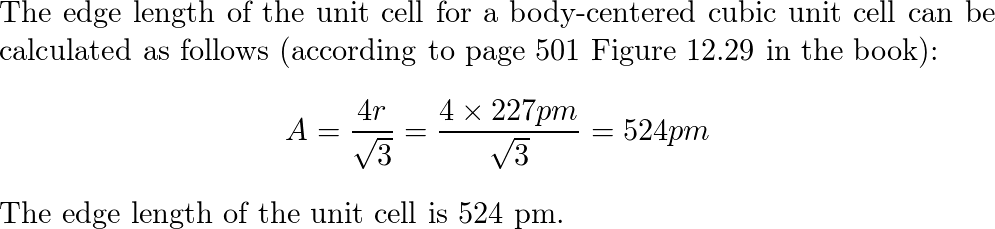
Potassium Adopts The Body Centered Cubic Unit Cell In Its Cr Quizlet

Electron Ion Collisions Fundamental Processes In The Focus Of Applied Research Sciencedirect

If The Edge Length Of A Kcl Cell Is 488 Pm What Is The Length Of Kcl Bond If It Crystallizes In The Fcc Structure

Calameo Ijp 6 2020
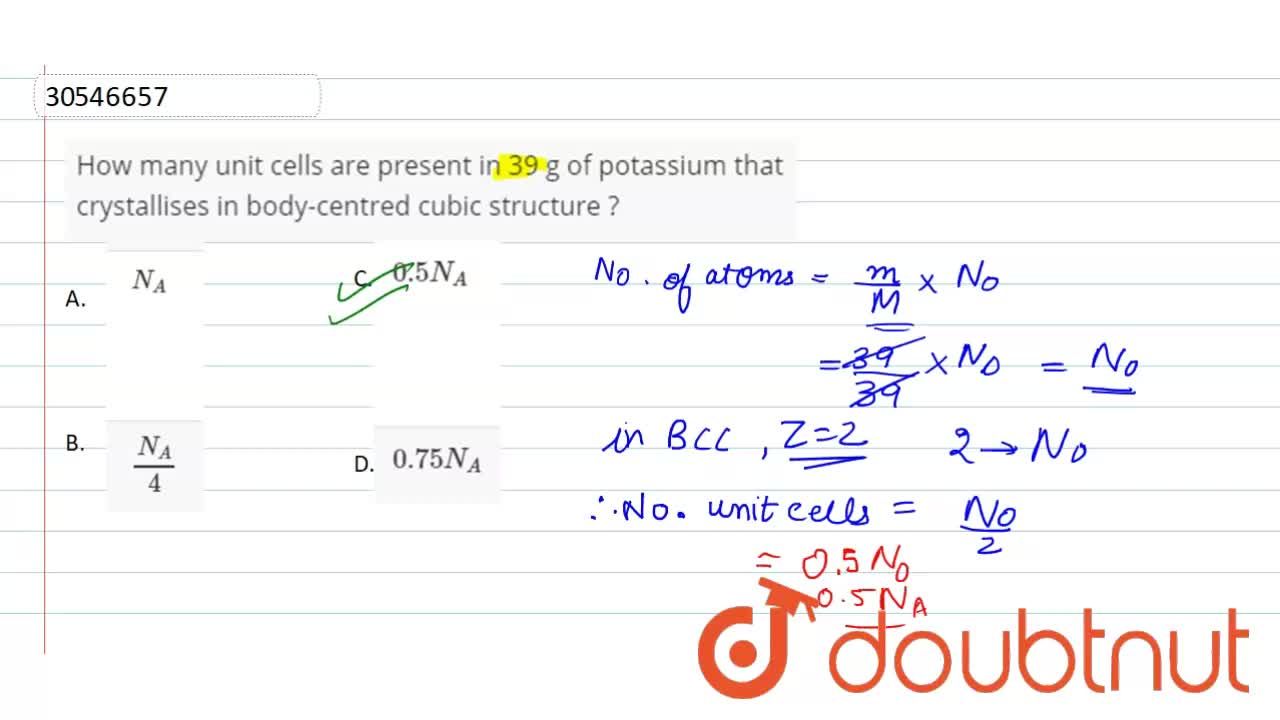
How Many Unit Cells Are Present In 39 G Of Potassium That Crystallises In Body Centred Cubic Structure
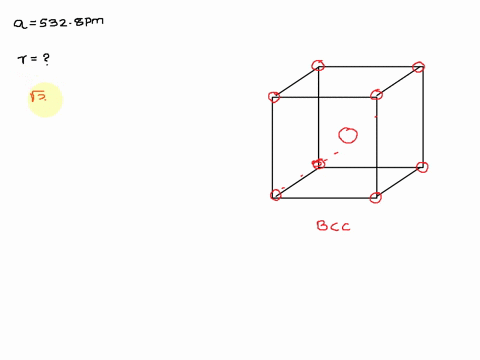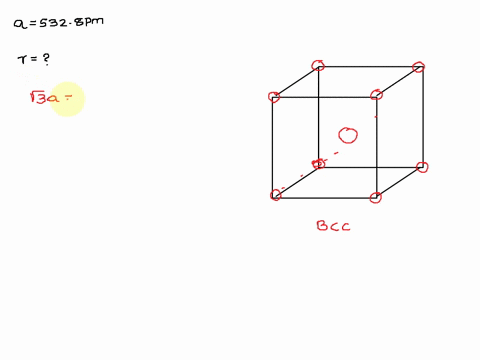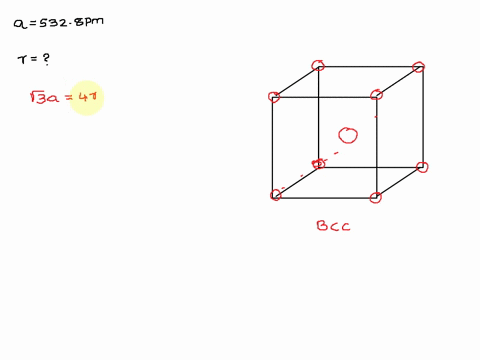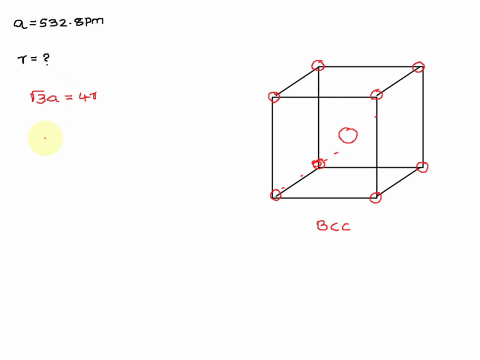
Solved The Unit Cell Of Potassium Is Bcc If Radius Of Potassium Atom Is 280 Pm Calculate Edge Length Of Unit Cell
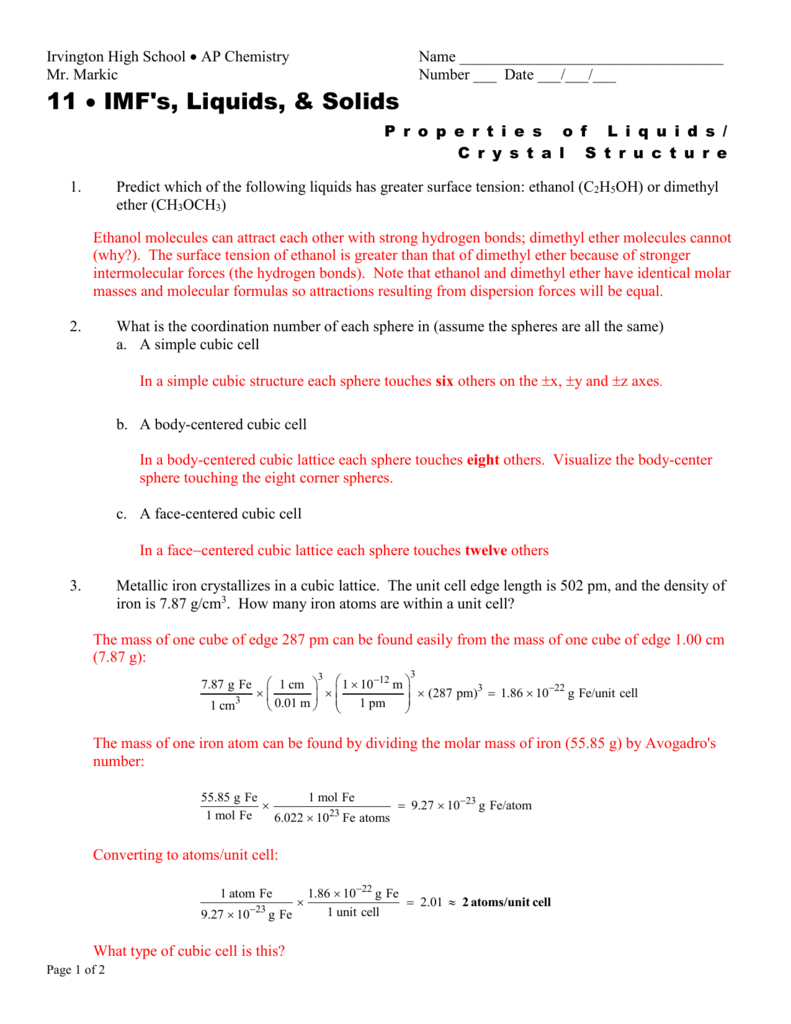
File Mr Markic S Chemistry

Pdf Development Of Novel Crystallizer Design And Optimization Tools For Solution Crystallization Robert Ingraham Academia Edu